Product show
Phosphorous Acid (H3PO3)
IDENTIFICATIONSynonyms: Orthophosphorous acid; phosphonic acid (IUPAC)
De: Phosphonsäure ES: Ácido fosfónico Fr:Acide phosphonique
CAS No.: 13598-36-2 EC No.: 233-663-1 RTECS No.: SZ6400000
Molecular Weight: 81.996
Molecular Formula: H3PO3
Structural Formula: 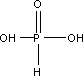
Density: 1.6510
M.P.: 73.6°C
B.P.: 200°C (decomposing at 180°C)
Physical & Chemical Properties: white solid, reducing, diprotic (not triprotic as its formula suggests) acid, hygroscopic and deliquescent.
Industrially and economically, phosphorous acid can be made by hydrolysis process of phosphorus trichloride (PCl3) or dimethyl phosphite (DMP) with water or hydrochloric acid: (1) PCl3 + 3 H2O →H3PO3 + 3HCl (2) DMP + 2H20 →H3PO3 + 2CH3OH. When using hydrochloric acid, methyl chloride gas will release.
1) The most important use of phosphorous acid is to produce phosphonates, for example ATMP, HEDP, PBTC which are used as scale inhabitor or corrosive inhabitor in water treatment, and PMIDA (CAS 5994-61-6) which is a very important intermediate of herbicide glyphosate.
2) Phosphorous acid is used for preparing phosphite salts, such as potassium phosphite, ammonium phosphite and calcium phosphite. These phosphite salts are widely applied in controlling downy mildew and late bright. Another important phosphite salt is lead phosphite dibasic which is used as PVC heat-stabilizer.
3) It is also used in chemical reactions as a reducing agent that is somewhat less vigorous than the related hypophosphorous acid.
4) Upon heating to around 200 °C, it is a convenient precursor to phosphine: 4H3PO3 → PH3 + 3H3PO4
| Grade II | Grade III | Grade IV | 70% Solution | |
|---|---|---|---|---|
| Phosphorous acid Purity | 99.0% min. | 98.5% min. | 98.5% min. | 70% min. |
| Chloride (Cl) | 10ppm max. | 0.01% max. | 0.01% max. | 0.01% max. |
| Iron(Fe) | 10ppm max. | 30ppm max. | 50 ppm max. | 10ppm max. |
| Sulfate(SO4) | 10ppm max. | 80ppm max. | 80 ppm max. | 80ppm max. |
| Phosphate (PO4) | 0.1% max. | 0.2% max. | 0.3% max. | 0.1% max. |
| Color,70% solution | colorless | colorless | colorless | colorless |
| Odor | odorless | odorless | odorless | odorless |
| Appearance | white crystals | white crystals | white crystals | clear colorless liquid |
1) 25kgs per PP woven bag with two PE inner bags, 19~27mt per 20’ FCL
2) For packaging 500 or 1000kgs/supper bag, maximum loading in 20’ FCL : 20000kg
3) 50kg per iron drum or plastic drum
4) 70% solution: 25kg or 250kg per plastic drum, or 1300kg per 1000L IBC

REACH Substance Certified Product for EU market
Class 8; UN 2834; PG III. Corrosive Acid, Dangerous.
R 22 Harmful if swallowed. R 35 Causes severe burns.
S 26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S 36/37/39 Wear suitable protective clothing, gloves and eye/face protection.
S 45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible).

